A study indicates that a COVID19 infection can lead to the autoimmune disease GuillainBarré syndrome (GBS) The immune system assaults the nerves, resulting in muscular weakness and, in rareA Houston woman who developed the rare neurological condition GuillainBarré syndrome (GBS) after receiving the onedose Johnson & Johnson COVID Covid could be an occasional trigger of GuillainBarré syndrome (GBS), a study has claimed The autoimmune disease, which can leave patients paralysed and in crippling pain, has also been linked to coronavirus vaccines in extremely rare cases Coronavirus itself is not listed as a known trigger of the condition by the National Health Service, even though other infections

J J Covid Vaccine May Pose Small Possible Risk Of Rare Neurological Syndrome Cdc Says Cbc News
Pfizer covid vaccine guillain barre syndrome
Pfizer covid vaccine guillain barre syndrome- Some people who got the Johnson & Johnson/Janssen COVID19 vaccine (the onedose vaccine) have developed a condition known as GuillainBarré syndromeIt's happened often enough that the FDA has The Johnson & Johnson coronavirus vaccine now comes with a warning on its label about an increased risk of nerve damage known as GuillainBarré syndrome (GBS) It has primarily been seen in men



Seven Cases Of Guillain Barre Noted After Covid 19 Vaccine In India Consumer Health News Healthday
to receive COVID19 vaccine is declining but that trend could easily shift if potential concerning sideeffects lead to more fear In this issue of Neurology, Marquez Loza and colleagues present a case of a patient with GuillainBarre syndrome (GBS) following COVID19 vaccination The patient developed typical GBS 10 days Scientists warn of GuillainBarre syndrome paralysis after Covid19 vaccination Numerous case reports of GuillainBarre syndrome paralysis after Covid19 vaccine have prompted scientists to warn that "all physicians" should be "vigilant in recognizing GuillainBarré syndrome in patients who have received the AstraZeneca vaccine our observations suggest COVID19 may precede GuillainBarré syndrome in rare cases, but a strong link is not likely, according to new data from an international consortium
Should people with a history of GuillianBarré Syndrome (GBS), an immune and nerve disease caused by viral and bacterial infections, get a COVID19 vaccine?We report the first case of Guillain–Barre Syndrome after receiving the second dose of the Pfizer COVID19 vaccine, in a 42yearold woman presenting with progressive ascending weakness and paresthesias Diagnostic workup demonstrated cytoalbuminologic dissociation on cerebrospinal fluid analysis with confirmatory evidence of early Clinical details of a participant in a Covid19 vaccine trial who developed GuillainBarré syndrome (GBS) after vaccination were published in Neurology Two cases of GBS, one in the active and one in the placebo arm, were reported in the Ad26COV2S (Johnson & Johnson) Covid19 vaccine trial data "Our patient in the vaccine arm did not have any clinical features
CDC guidance says that people who have previously had GuillainBarre syndrome may receive the COVID19 vaccine That isn't to discount CruzEsteves' personal experience — or the way the diagnosis has affected her life "I haven't seen my daughter for 5 Reports of GBS after COVID19 vaccination risk raising fears about the safety of the vaccines, particularly among those who are hesitant or fearful about being vaccinated While the association of viral infections with GBS has long been recognized, whether vaccinations can increase the risk of GBS is less certain The FDA has released a new warning that the onedose Johnson & Johnson COVID19 vaccine could increase people's risk of developing GuillainBarré syndrome, a rare neurological disorder Here's




Coronavirus Vaccine Side Effects Guillain Barre Syndrome How Risky Is Guillain Barre Syndrome The Nervous System Disorder Identified As A Covid 19 Vaccine Side Effect




U S Adds Warning To J J Covid 19 Vaccine Over Small Possible Risk Of Guillain Barre Syndrome Ktla
On 13 and July 21, the COVID19 subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS) met virtually to discuss rare reports of GuillainBarré Syndrome (GBS) following vaccination with the Janssen and AstraZeneca COVID19 vaccines Both vaccines use an adenovirus platform as their backboneWe feel you there, too Here London A Covid19 infection may prompt autoimmune disorder, the GuillainBarre syndrome (GBS), finds a study In the disorder, a person's immune system attacks the nerves, causing muscle weakness and occasionally, paralysis The condition is triggered by an acute bacterial or viral infection and can last for weeks or several years



Us Warns Of Link Between J J Vaccine And Guillain Barre Syndrome World English Edition Agencia Efe




Clinicians Urged To Spot Guillain Barre Syndrome After Covid 19 Vaccines
100 cases of GuillainBarré syndrome have been reported among people who received J&J's vaccine, the FDA said in a statement, and the FDA changed its fact sheets on the vaccine for providers andHowever, individual cases and population cohorts should be scrutinised, in order to ensure the GuillainBarré syndrome (GBS) will be listed as a very rare side effect of COVID19 Vaccine Janssen and a warning will be included in the product information to raise awareness among healthcare professionals and people taking the vaccine GBS is a rare neurological disorder in which the body's immune system damages nerve cells which can result in pain, numbness and




What Covid Vaccines Are Related To The Guillain Barre Syndrome What Are The Cdc Guidelines As Com
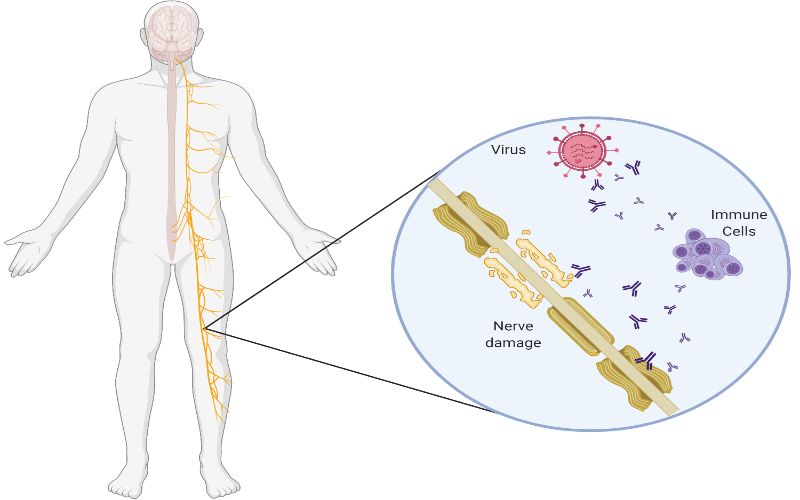



No Association Between Covid 19 And Guillain Barre Syndrome Ucl News Ucl University College London
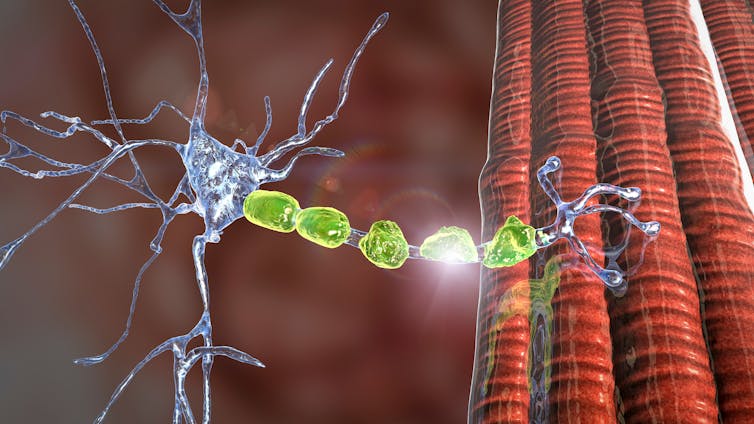
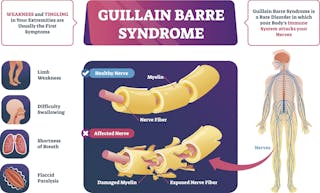
GuillainBarré syndrome (GBS) is a rare immunemediated disorder of the peripheral nerves Although its cause is not fully understood, the syndrome often follows infection with a virus or bacteria, although in rare occasions, vaccination may precede GBS We describe a case of a 62yearold woman who presented with paraesthesia and progressive weakness of both lower limbs Johnson & Johnson's beleaguered Covid19 vaccine may be associated with a small increased risk of Guillain–Barré syndrome, a rare but Regulators say the benefits of the vaccine still outweigh any risks GuillainBarré syndrome (GBS) is a rare neurological disorder in which the immune system attacks the nerves It can cause muscle weakness and sometimes paralysis People usually recover from it, but it can lead to hospitalization and, sometimes, permanent damage to nerve cells




Can The Covid Vaccine Cause Guillain Barre Syndrome



Cureus Neurological Complications Of Covid 19 Guillain Barre Syndrome Following Pfizer Covid 19 Vaccine
This week, the FDA announced that it would attach a warning to the Johnson & Johnson COVID19 vaccine pertaining to its very slightly elevated risk association with GuillainBarré syndrome, a rare neurological disorder The announcement came after approximately 100 suspected cases were identified among the more than 12 million people who've received the Albert Shaw, MD, PhD, a Yale Medicine infectious diseases specialist, notes that the incidence of GBS after the Johnson & Johnson vaccine, as with other reported associations of GBS with other vaccines, has been very rare Consequently, "It should not prevent people from receiving the vaccine, as the benefits of the vaccine in preventing serious illness or death fromGuillainBarré syndrome (GBS) is a rare autoimmune disease in which your body's immune system damages your nerves It causes muscle weakness and sometimes paralysis Symptoms are mostly temporary and typically last anywhere from a few weeks to several years



Avisan De Un Vinculo Entre Janssen Y Sindrome Guillain Barre



Guillain Barre Syndrome Johnson And Johnson Vaccine Fit Increase Risk Of Nerve Disorder Us Fda Warn c News Pidgin
The study emerged after reports stated that those inoculated with the AstraZeneca vaccine might be exposed to the GuillainBarré syndrome The list of repercussions caused by COVID has added another illness as a new study suggests possible links between the Coronavirus and the GuillainBarré syndrome (GBS)Risk of confirmed GuillainBarre syndrome following receipt of monovalent inactivated influenza A (H1N1) and seasonal influenza vaccines in the Vaccine Safety Datalink Project, 0910 external icon Am J Epidemiol 12 Jun 1;175(11) Epub 12 May 11 Covid could be an occasional trigger of GuillainBarré syndrome (GBS), a study has claimed The autoimmune disease, which can leave patients paralysed and in crippling pain, has also been linked




Guillain Barre Syndrome Associated With Sars Cov 2 Nejm
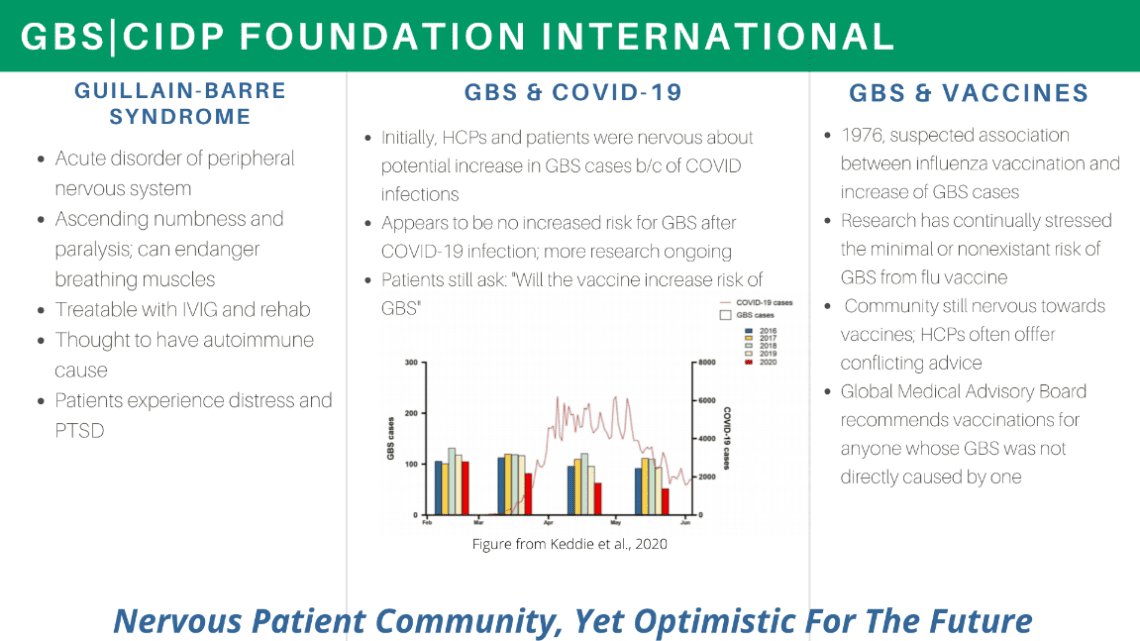



Gbs Cidp Foundation Staff Patients Collaborate With Fda Gbs Cidp Foundation International
What the FDA says about the J&J vaccine and GuillainBarré syndrome Nearly 13 million doses of the Johnson & Johnson COVID19 vaccine have been administered in the US, compared with 185 Learning points Though there is currently no evidence of any association between COVID19 vaccination and GuillainBarré syndrome (GBS) incidence, it is important to keep a high index of suspicion and to report any potential side effects or Two reports detailed cases of an unusual variant of GuillainBarré syndrome (GBS) associated with the AstraZeneca adenovirus vector COVID




Fda Adds Guillain Barre Syndrome Warning To J J Covid Shot Politico



Fda Warns About J J Covid Vaccine And Guillain Barre Syndrome Risk Health Com
A haywire immune system, attacking healthy nerves, causes the disorder US health authorities added a warning to the label of J&J's Covid19 vaccine after finding 100 reports The Therapeutic Goods Administration (TGA) yesterday revealed there have been six reports of GuillainBarré syndrome in Australia following the AstraZeneca COVID vaccine This is an autoimmune Researchers at Rutgers University have reported the first instance of COVID19 triggering a recurrence of GuillainBarré Syndrome, a rare and potentially deadly disorder in




Hyderabad Covid 19 Vaccine Does Not Cause Gbs Say Doctors Hyderabad News Times Of India



Brazil Also Reports Guillain Barre Cases After Vaccination Agencia Brasil
Covid could be an occasional trigger of GuillainBarré syndrome (GBS), a study has claimed The autoimmune disease, which can leave patients paralysed and in crippling pain, has also been linked to coronavirus vaccines in extremely rare cases The Food and Drug Administration warned on Monday that Johnson & Johnson's coronavirus vaccine can lead to an increased risk of a rare neurological condition known as GuillainBarré syndrome It's all about the Janssenmade Johnson & Johnson (J & J) COVID19 vaccine and GuillainBarré Syndrome (GBS) If you saw that and thought a) Whoa and b) Whaa??




70q7symtp1eom



New Warning For Astrazeneca Covid 19 Vaccine Mims Online
CDC 100 People Vaccinated With J&J COVID Shot Developed GuillainBarre Syndrome Start your unlimited Newsweek trial Continue Reading Show full articles without "Continue Reading" button for {0 In December , two vaccines have been approved in the United States for the prevention of COVID19 We report a case of GuillainBarre Syndrome (GBS) after receiving the first dose of Pfizer COVID19 vaccine Keywords neurologic complications, guillainbarre syndrome (gbs), covid19 vaccine Introduction London A COVID19 infection may prompt autoimmune disorder, the GuillainBarre syndrome (GBS), finds a studyIn the disorder, a person's immune system attacks the nerves, causing muscle weakness and occasionally, paralysis The condition is triggered by an acute bacterial or viral infection and can last for weeks or several years




What Is Guillain Barre Syndrome Read On About The Rare Syndrome That Some Covid 19 Patients Are Developing



What Is Guillain Barre Syndrome Fda Warns Of Increased Risk Of Nerve Disorder After Johnson Johnson Vaccine Nbc Chicago
EMA European Medicines Agency recently investigated the cases of GuillainBarre syndrome (GBS) following Covid vaccine AstraZeneca (AZ) and have issued a warning to raise awareness of healthcare professionals and the public about GBS cases following Covid vaccinations and recommended revising the product information for Covid vaccine AZ 1We report a case of GuillainBarré syndrome (GBS) occurring soon after the first dose of Vaxzevria (previously known as COVID19 vaccine AstraZeneca) Thus far, there has been no evidence of an increased risk of GBS resulting from either COVID19 infection nor from COVID19 vaccines; Any risk of GBS or that people with past GBS should avoid either the PfizerBioNTech COVID19 Vaccine or the Moderna COVID19 Vaccine is not listed in the FDA authorized package insert for either




Guillain Barre Syndrome Linked To Johnson Johnson Covid Vaccine




Who On Reports Of Guillain Barre Gbs After Covid 19 Vaccines Medical Laboratory Observer
Most people fully recover from GBS," a CDC spokesperson told CNN "Reports of GBS after receipt of the J&J/Janssen COVID19 Vaccine in the Vaccine Adverse Event Reporting System (VAERS) are rare It is intended to tell you to pay attention to your body whether you get the vaccine, get COVID, or do nothing Because the scary fact is To our knowledge, this is the first study assessing safety of messenger RNA COVID19 vaccine in previously diagnosed cases of GBS In this cohort study, which included 702 patients, only 1 needed short medical care for relapse of previous syndrome, which represents a minimal risk The study has limitations




Johnson Johnson S Covid 19 Vaccine Has Another Problematic Side Effect




No Proof Covid Vaccines Can Trigger Guillain Barr Syndrome Community Healthcare System
US health regulators have warned that Johnson & Johnson 's Covid19 vaccine may be related to an increased risk of the rare neurological disorder known as Guillain–Barré syndrome The risk of thisCase of GuillainBarré syndrome following COVID19 vaccine GuillainBarré syndrome (GBS) is a rare immunemediated disorder of the peripheral nerves Although its cause is not fully understood, the syndrome often follows infection with a virus or bacteria, although in rare occasions, vaccination may precede GBS What is GuillainBarre syndrome, and can you get it from the J&J vaccine?




J J Covid Vaccine May Pose Small Possible Risk Of Rare Neurological Syndrome Cdc Says Cbc News



Vaccine Guillain Barre Syndrome A New Rare Side Effect Associated With Astrazeneca Sortiraparis Com
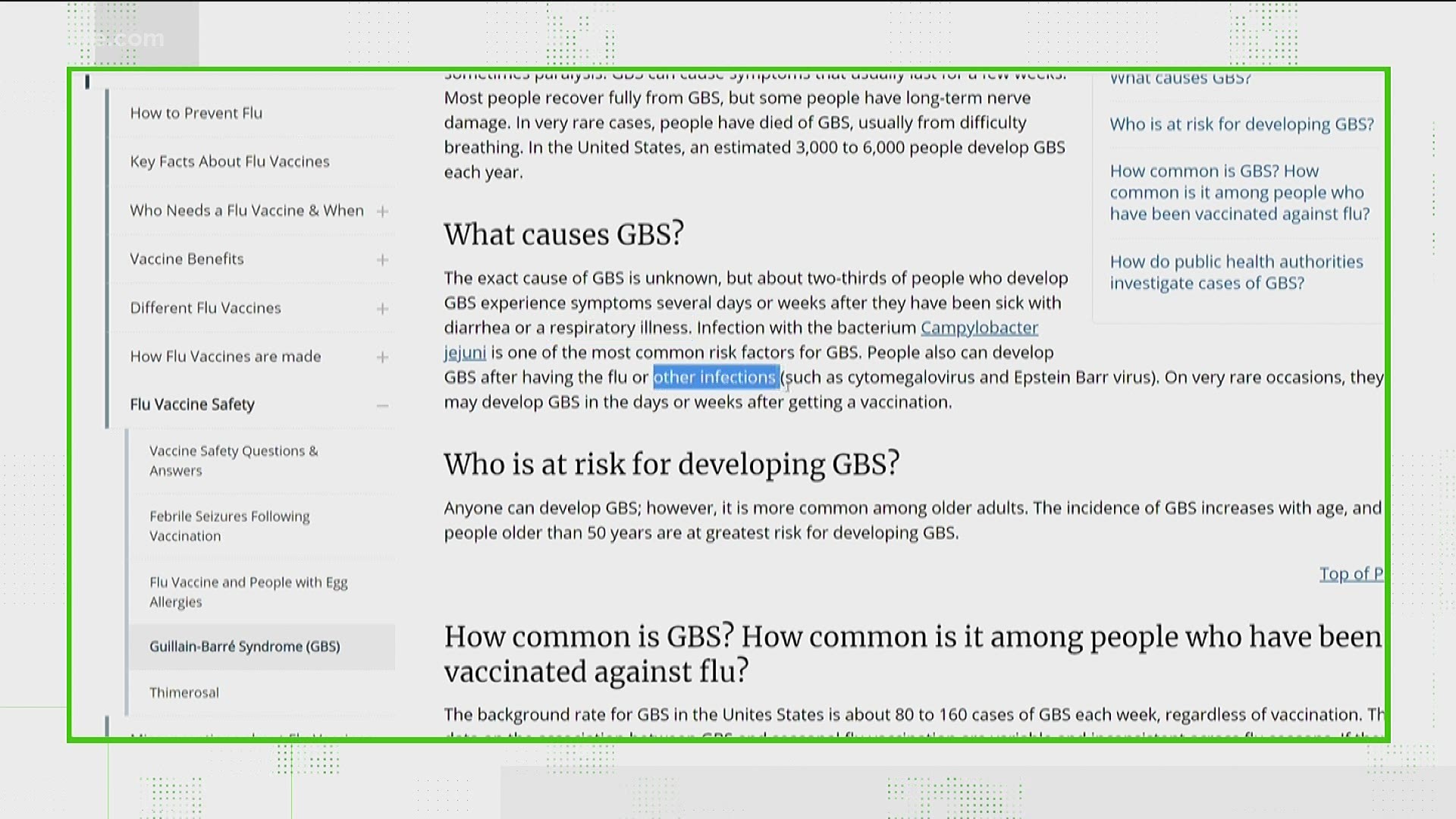



J J Warning And The Guillain Barre Syndrome 11alive Com




J J Covid Vaccine Warning Fda Adds Info On Rare Reaction Risk 13newsnow Com
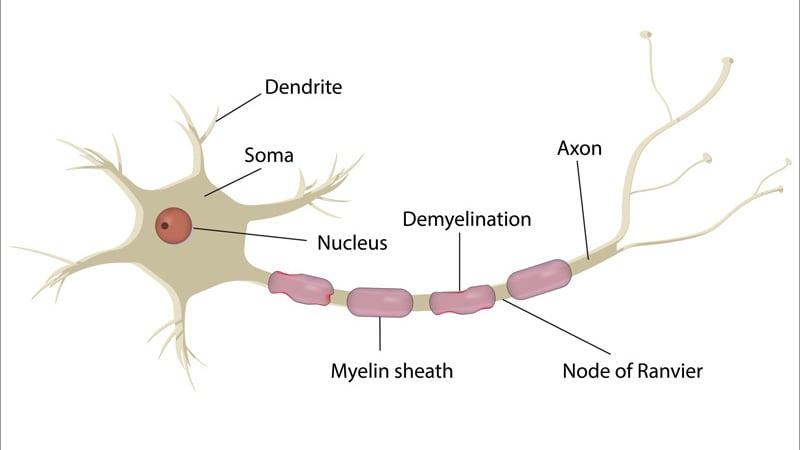



First Case Of Covid 19 Presenting As Guillain Barre Reported



Rare Cases Of Guillain Barre Syndrome Reported In Janssen Covid 19 Vaccine



What Is The Guillain Barre Syndrome Affecting Covid 19 Patients




What To Know About Guillain Barre And The Covid Vaccine Cleveland Clinic




J J Astrazeneca Explore Covid Vaccine Changes Due To Clots Wsj Coronavirus Pandemic News Al Jazeera




Explained What Is Guillain Barre Syndrome Why It S Being Linked To J J Vaccine




Fda Johnson Johnson Covid 19 Vaccine Tied To Guillain Barre Syndrome




People With Guillian Barre Syndrome Can Take The Covid Vaccine 9news Com




Seven Cases Of Guillain Barre Noted After Covid 19 Vaccine In India Consumer Health News Healthday




Astrazeneca Covid Vaccine Linked To Rare Neurological Disorder In India Uk India News The Indian Express
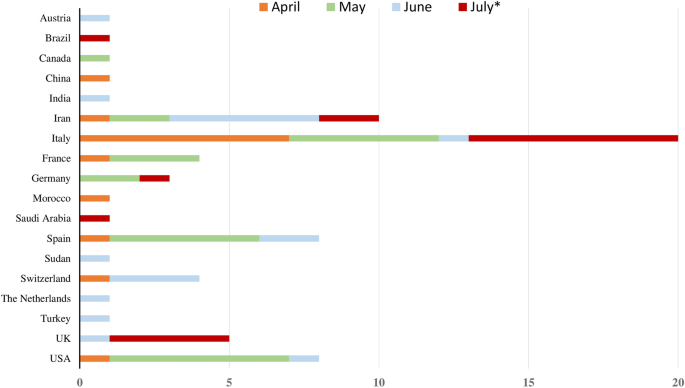



Guillain Barre Syndrome Spectrum Associated With Covid 19 An Up To Date Systematic Review Of 73 Cases Springerlink



Guillain Barre Syndrome In Covid 19 Vaccine Trial Does Not Imply Causality Experts Say



The Johnson Johnson Vaccine And Guillain Barre Syndrome What Michigan Needs To Know Bridge Michigan




Nerve Disorder Listed As Very Rare Side Effect Of Astrazeneca Jab France 24



Johnson Johnson Covid Vaccine And Guillain Barre Everything To Know




Guillain Barre Syndrome The Lancet




Rutgers Reports First Instance Of Covid 19 Triggering Recurrent Guillain Barre Syndrome Rutgers University



Could The Astrazeneca Vaccine Cause Guillain Barre Syndrome We Don T Know Yet But There S Minimal Cause For Concern




Dear Pandemic Q Does The Covid 19 Vaccine Cause Guillain Barre Syndrome A The Janssen Vaccine May Increase The Risk Of Guillain Barre Syndrome Gbs But We Don T Yet Know For Sure Even If



Fda Adds Guillain Barre Syndrome Warning To J J Covid Vaccine Axios



Covid Help Desk I Ve Had Guillain Barre Syndrome With A Flu Shot What Is My Risk With The Vaccine



What Are The Symptoms Of Guillain Barre Syndrome Should I Still Get The J J Vaccine Marketwatch
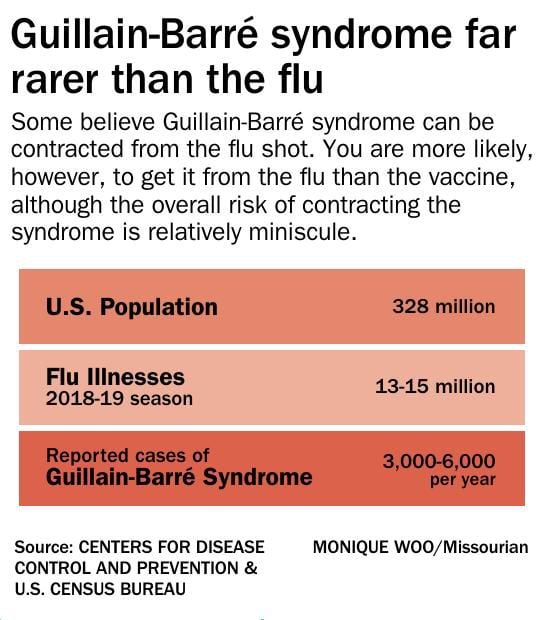



Guillain Barre Syndrome What Is It And Why Should You Care News Columbiamissourian Com



Eu Reviews Reports Of Rare Nerve Disorder After Astrazeneca S Covid 19 Shot Reuters



What Do You Need To Know About The Guillain Barre Disease And Johnson And Johnson Vaccines Technology News Firstpost



Could The Astrazeneca Vaccine Cause Guillain Barre Syndrome We Don T Know Yet But There S Minimal Cause For Concern




Guillain Barre Syndrome The Lancet




What Is Guillain Barre Syndrome And Its Symptoms Miami Herald



Guillain Barre Syndrome News Articles Etc European Pharmaceutical Review



Rare Neurological Disorder Guillain Barre Syndrome Linked To Covid 19



Fda Will Announce Rare Incidence Of Guillain Barre Linked To Johnson Johnson Vaccine




Johnson Johnson Vaccine Increases Risk Of Rare Nerve Disorder Us Fda Warns



Statement Of The Who Global Advisory Committee On Vaccine Safety Gacvs Covid 19 Subcommittee On Reports Of Guillain Barre Syndrome Gbs Following Adenovirus Vector Covid 19 Vaccines Paho Who Pan American Health Organization




Que Es El Sindrome De Guillain Barre Cuya Vinculacion Con La Vacuna De Janssen Estan Investigando



Us Warns Of Link Between J J Vaccine And Guillain Barre Syndrome World English Edition Agencia Efe



Fda Adds Warning To J J Covid 19 Vaccine After Gbs Cases Cgtn




Guillain Barre Syndrome And The J J Vaccine What To Know And What Are The Risks Wsj




Case Reports Describe Unusual Guillain Barre Variants Following Covid 19 Vaccination




Astrazeneca S Covid 19 Shot Joins The List Of Vaccines Flagged For Rare Guillain Barre Syndrome Fiercepharma




Covid Vaccine Updates Fda Warns Of Rare Guillain Barre Syndrome Reaction To Johnson Johnson Coronavirus Vaccine Abc7 New York



Rare Neurological Disorder Guillain Barre Syndrome Following Covid 19 Vaccination




Covid 19 Vaccine Exhibits Minimal Risk For Recurrent Guillain Barre Syndrome




The Fda Warns That Johnson And Johnson Covid Vaccine May Be Linked To Guillain Barre Syndrome Wusa9 Com



Cdc Vaccine Panel To Discuss J J S Covid Vaccine Guillain Barre Syndrome Bloomberg



Guillain Barre Syndrome News Research And Analysis The Conversation Page 1




Study Finds No Link Between Covid 19 Guillain Barre Syndrome Cidrap



Single Dose Covid Vaccine Linked With Rare Cases Of Guillain Barre Syndrome Pulse Today



Rarest Of Covid Vaccine Reactions Guillain Barre Syndrome Whyy



Fda Adds Guillain Barre Warning To J J Vaccine What To Know Time




Un Adolescente Es Diagnosticado De Guillain Barre Semanas Despues De La Primera Vacuna Covid Children S Health Defense
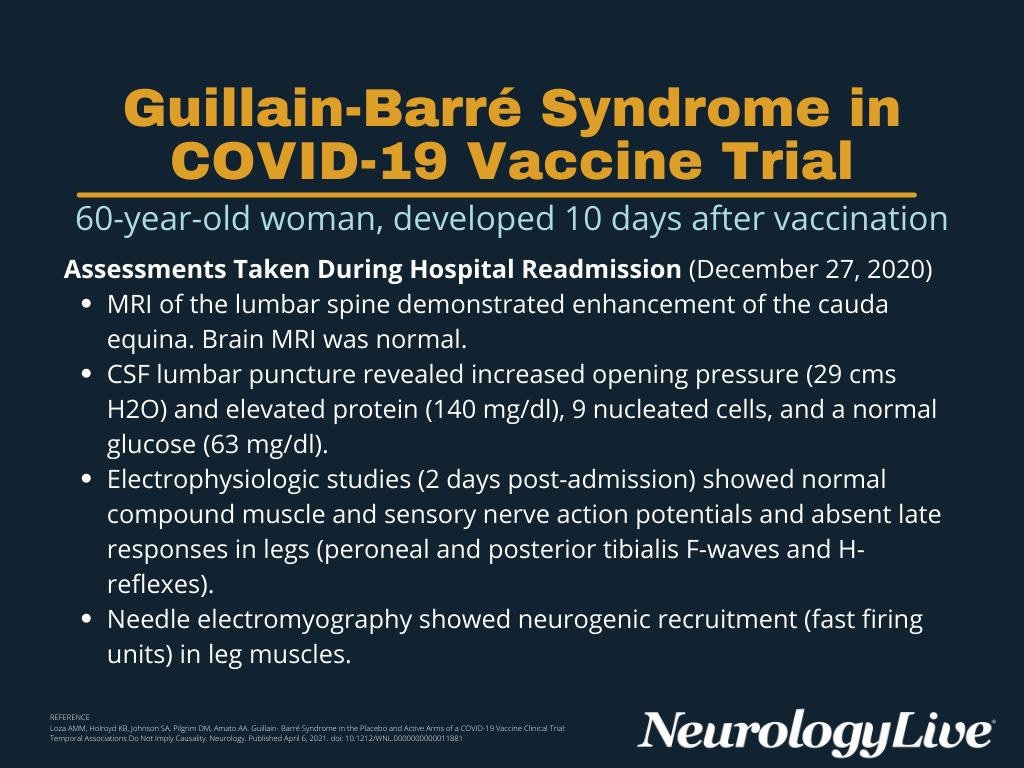



Guillain Barre Syndrome Reported In Active Placebo Groups Of Covid Vaccine Trial




Unusual Variant Of Guillain Barre Syndrome Linked To Covid Vaccines Medpage Today



Fda Warns Of Possible Rare Association Between Johnson Johnson Vaccine And Guillain Barre Syndrome




Stakeholder Call Guillain Barre Syndrome Gbs Covid 19 Vaccine Updates 7 15 21 Youtube




Guillain Barre Syndrome And Johnson Johnson Vaccine What You Need To Know The Independent



La Ema Pide Estar Alerta A Signos Del Sindrome De Guillain Barre Con Astrazeneca




Guillain Barre Syndrome A Sudden Paralysis Of The Arms And Legs Science In Depth Reporting On Science And Technology Dw 13 07 21




Risk Of Guillain Barre Syndrome After Seasonal Influenza Vaccination And Influenza Health Care Encounters A Self Controlled Study The Lancet Infectious Diseases




What Is Guillain Barre Syndrome The Rare Condition That The Fda Have Linked To The Johnson Johnson Vaccine As Com




Fda Warns About Post Covid Vax Guillain Barre Syndrome Medpage Today




Fda Expected To Issue New Warning On J J Covid Jab Business And Economy News Al Jazeera



Covid 19 May Trigger Recurrent Guillain Barre Syndrome Episodes A Case Study Shows




Guillain Barre After Covid 19 Vaccine Case Report Medpage Today




Sindrome De Guillain Barre Que Es Y Como Se Relaciona Con La Vacuna The New York Times




Q A Guillain Barre Syndrome In Patients With Covid 19 Requires More Research




What To Know Guillain Barre Syndrome And The J J Vaccine




Janssen Covid 19 Vaccine Fact Sheet Updated With Guillain Barre Syndrome Warning




Tga Investigates Guillain Barre Syndrome Diagnoses After Astrazeneca
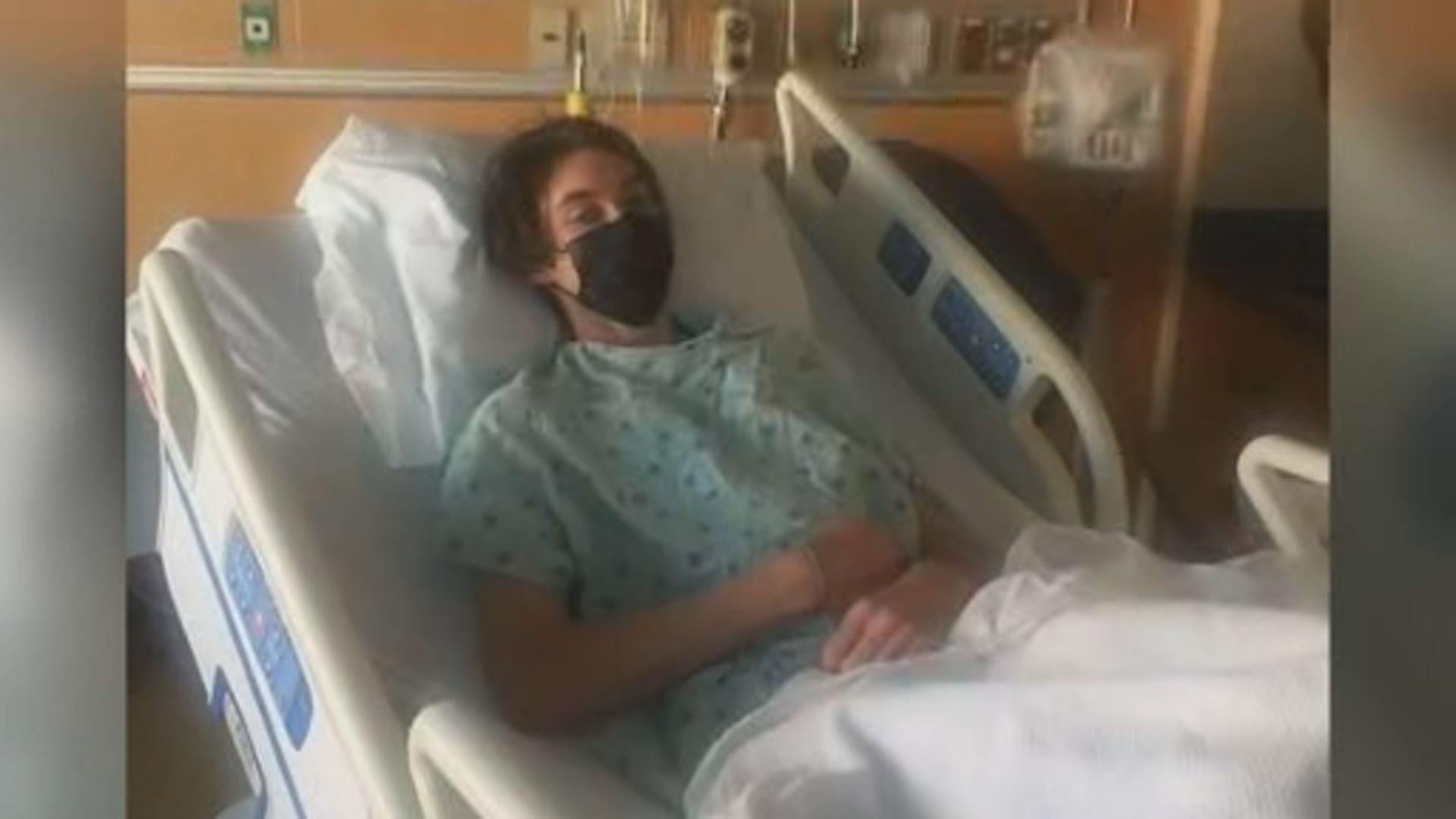



Local Teen Diagnosed With Guillain Barre Syndrome Questions Covid 19 Vaccine After Receiving First Dose




Guillain Barre Syndrome And Its Variants As A Manifestation Of Covid 19 A Systematic Review Of Case Reports And Case Series Journal Of The Neurological Sciences




Reports Of Guillain Barre Syndrome Gbs After Johnson And Johnson Covid 19 Vaccination




Rare Cases Of Gbs May Occur After Janssen And Astrazeneca Vaccines Who Panel



0 件のコメント:
コメントを投稿